SERIALIZATION AND TRACK-AND-TRACE SOLUTIONS FOR PHARMACEUTICAL WHOLESALERS
COMPLIANCE AND WAREHOUSE MANAGEMENT
In today’s serialized world, wholesalers and distributors must be equipped to verify all inbound products at the packaging level, including GTIN and serial numbers. To achieve this, many wholesalers and distributors are equipped with handheld devices that are integrated into the manufacturer’s L4 or into a serial-number management system.
Wholesalers and distributors also need to accept both serialized and non-serialized products, which means employees have multiple inbound processes to follow.

Track-and-Trace Solution
CHALLENGES WHOLESALERS AND DISTRIBUTORS FACE
- Complying with national track-and-trace regulations
- Conducting serialized rework operations in warehouse
- Managing product returns
- Optimizing picking processes
- Digitalizing paper-based processes
Serialization Solution
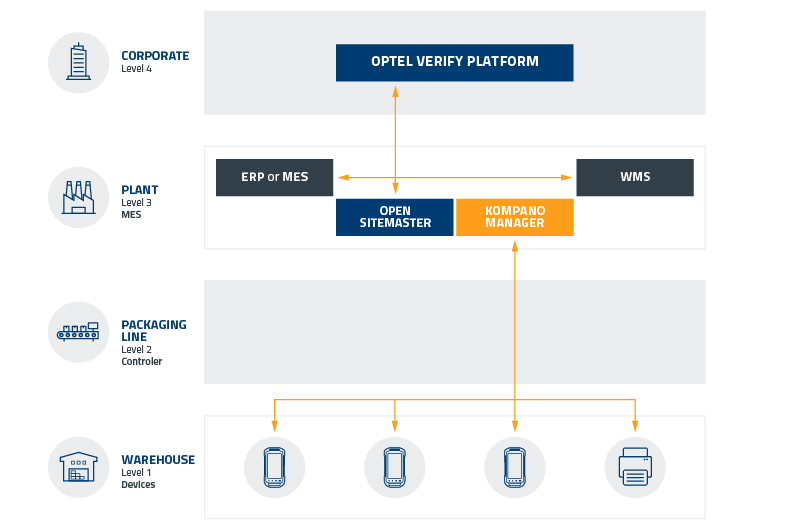
OPTEL’S TECHNOLOGY FOR WAREHOUSES AND DISTRIBUTORS
Choosing an L4 serialization provider is a daunting task that should not be taken lightly. OPTEL’s Verify Platform seamlessly circumvents the complexities of partner integration, decreases implementation times and offers value-added business benefits that deliver competitive advantages to wholesalers and distributors.
WHAT OPTEL’S TRACEABILITY SOLUTIONS CAN DO FOR YOUR BUSINESS

FLEXIBLE SOLUTIONS TO MEET YOUR NEEDS
Case StudyCATEGORIES THAT MAY INTEREST YOU

Pharmaceutical
Track-and-trace systems for the pharmaceutical industry
Manufacturers
Track-and-trace solutions to help comply with all upcoming regulations
CMOs and CPOs
Serialization solutions to help achieve compliance
3PLs and Repackagers
Achieving compliance without disrupting the supply chain
Hospitals and Pharmacies
Solutions for healthcare providers and pharmacies
Medical Devices
Turnkey vision inspection and traceability solutions
