CartonTracker
Automated serialization solution with great stability for printing and inspection, and that has an integrated ejection system
Driven mainly by compliance requirements from governments, companies need to implement mass serialization, inspect and authenticate products, and provide traceability records to prove compliance and protect their brand.
OPTEL has become the recognized global leader in track-and-trace technology, with more than 35 years of experience in the pharmaceutical, food and beverage, and agrochemical industries and more than 6,000 solutions deployed around the world.
Our stack of technologies includes powerful vision systems, turnkey serialization and aggregation hardware and software solutions.


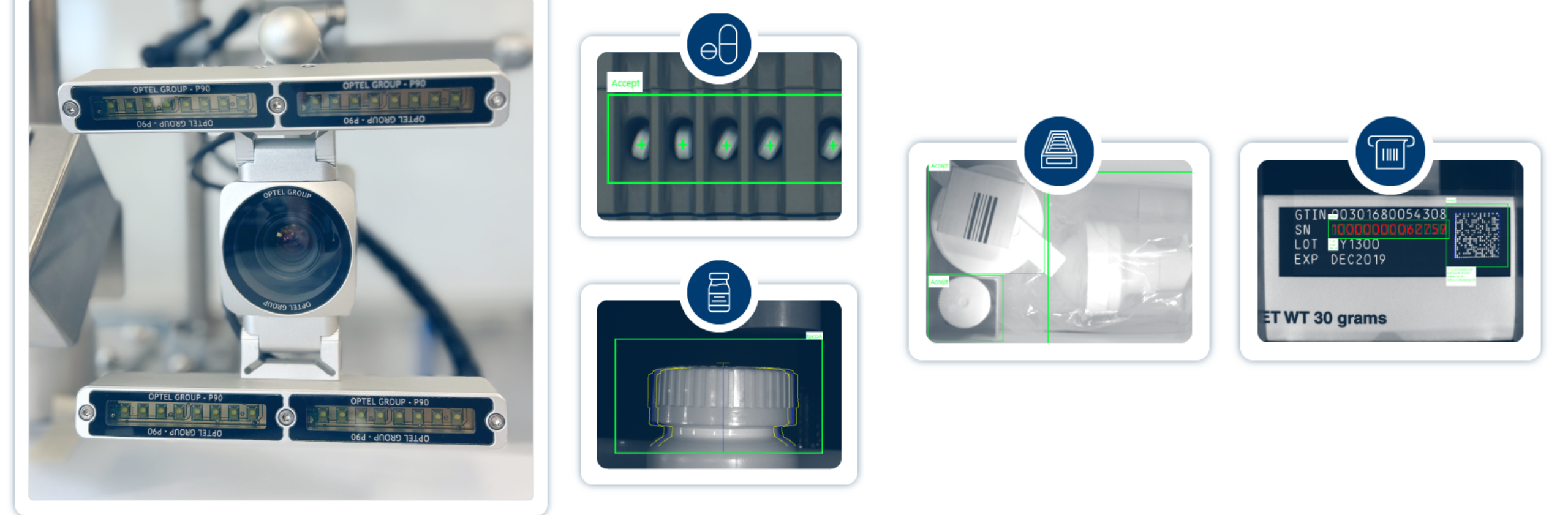

Vision Systems
Ensure pharmaceutical compliance and product quality with OPTEL’s AI-powered vision systems. Achieve 100% inspection and seamless integration.

Serialization
Serialization is the first step in the traceability journey. OPTEL delivers scalable pharma solutions for global compliance and end-to-end visibility.

Aggregation
Aggregation takes you beyond unit-level traceability to see the whole picture

Site-Level Solutions (L3)
Manage your plant-level serialization operations with the latest technology

VerifyBrand™ for the Pharmaceutical Industry
VerifyBrand™ is a serialization software helping pharmaceutical manufacturers to comply with traceability regulations.

Warehouse Solution
Manage your distribution and warehouse serialization process

Line Automation
Production Line Automation Solutions: from the case packer to the end-of-line Cobot Palletizer, discover OPTEL’s high performing and turnkey automated line solutions.

CartonTracker
Automated serialization solution with great stability for printing and inspection, and that has an integrated ejection system

CountSafe (Slat Counter)
Automated visual inspection solution designed to detect wrong color, missing items, broken products, foreign objects, etc.

Cobot Palletizer
Automated end-of-line solution with a cobot palletizer for the pharmaceutical industry

PackStation (SAP)
Semi-automatic solution designed to read serial numbers and add them to shipping cartons
















































































































