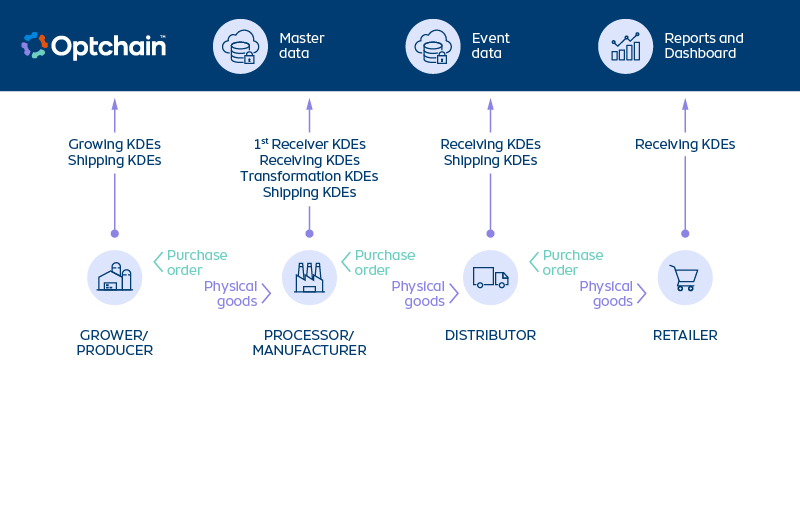
In November 2022, the US Food and Drug Administration (FDA) issued its final ruling on the Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule) under the Food Safety Modernization Act (FSMA) Section 204(d).
Commonly known as FSMA 204, this regulation is a key component of the FDA’s New Era of Smarter Food Safety Blueprint. It is also the result of sweeping reforms in the United States regarding food safety under the FSMA, which was passed by Congress in December 2010 and signed into law by President Barack Obama in January 2011. However, it took several years for the FDA to finalize the FSMA’s regulations and how compliance would be enforced.
What does this mean for agrifood companies, including producers, processors, distributors, retail chains, restaurants, etc.? Let’s take a closer look at the FSMA framework and what FSMA 204 means for their businesses.