WHAT IS 3T DOCUMENTATION?
In the pharmaceutical supply chain, all trading partners are licensed either by the FDA or a State Board of Pharmacy. Members of the supply chain can buy or sell products only to those parties with active licenses. This effectively means that each partner in the supply chain is regularly evaluating the licenses of its customers and suppliers.
Along with the licenses, the seller must provide standardized transaction documents to their customers for each shipment. These documents include:
TRANSACTIONAL INFORMATION (TI)
This document describes the specifics of the current transaction. It includes:
- The name of the product
- Dosage and strength of the product
- NDC (National Drug Code)
- Lot number
- Date of transaction
- Date of shipment
- Container information
- Number of containers
If more than 24 hours have passed since the transaction date, the Date of shipment is to be included.
TRANSACTIONAL HISTORY (TH)
The TH outlines all the transactions a product has gone through, starting from the manufacture. This is essentially a bunch of TIs attached together for reviewing a product’s entire supply chain journey.
TRANSACTIONAL STATEMENT (TS)
The TS is a declaration confirming that the seller:
- Is authorized and registered
- Has received the product from a registered and authorized party
- Did not purposefully change the transaction history
- Did not purposefully ship any ineligible, counterfeit, or suspicious product
- Has acknowledged the transaction statement and information from the previous seller in the supply chain
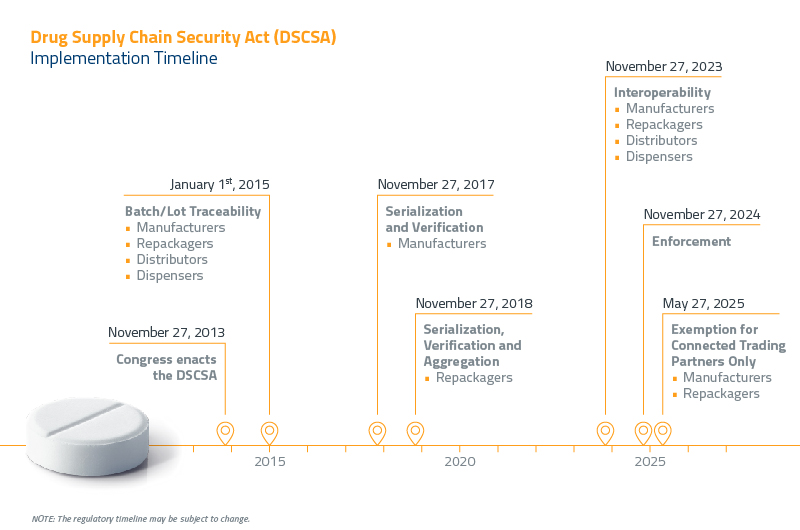
Beginning November 27, 2023, only TI and TS will be required. However, TI requirements will be extended to include product identifier data for all packaging levels. Since the National Drug Code (NDC) and lot number are already in the TI data, this means only the serial numbers and expiration dates will need to be added.
WHAT ARE THE SERIALIZATION REQUIREMENTS UNDER DSCSA?
By November 27, 2020, all key players in the pharma supply chain, including repackagers, manufacturers, distributors, and dispensers, must generate, authenticate, and verify serial numbers for all packages traveling through the chain.
This means all packaging levels must have complete serialized product identifiers. As for the serial numbers themselves, the Standardized Numeric Identifiers (SNI) must be generated as per the 2009 FDA directive. SNIs consist of the 20-character NDC (National Drug Code), batch/lot number, Global Trade Item Number (GTIN), and expiration date. Also, the smallest saleable unit needs to be packaged with a 2D Barcode Matrix with human-readable text. Shipping cases also require an SNI with a Serial Shipping Container Code (SSCC).
WHAT ARE THE AGGREGATION REQUIREMENTS UNDER DRUG SUPPLY CHAIN SECURITY ACT (DSCSA)?
To help comply with verification requirements and to easily share data with trading partners, the FDA encourages the adoption of aggregation. What is aggregation? With both drugs and transport units (bundle, pallets, and containers) serialized, a system of hierarchical serialization is achieved. In a bundle of cases carrying bottles, for instance, each bottle features a unique serial number, as does the case. This forms an aggregate relationship between the case and the bottle.
REASON FOR AGGREGATION
The hierarchical serialization makes it possible to identify items accurately in the supply chain and to detect suspicious activities. However, that’s not it. Aggregation also confers time and cost-saving benefits on pharmaceutical manufacturers and packagers. For instance, repackagers don’t need to spend time scanning every smallest saleable unit, and can only scan the parent. This, naturally, results in substantial time savings.
REQUIREMENT AND TIMELINE
Pharmaceutical manufacturers and supply chain members must achieve complete, interoperable track-and-trace aggregation by November 27, 2023. If you have not chosen an aggregation system yet, contact OPTEL for a full line evaluation and system recommendation.
HOW TO MANAGE DATA EXCHANGE WITH TRADE PARTNERS
Once the DSCSA is in full effect, serialization compliance will affect your business far beyond the production line. In reality, you will have to exchange data with different organizations, each with its own system and data formats.
To build such an interoperable system, you’ll need:
- Processes and systems to inspect the product down to the item-level
- Systems to reliably process transaction history
- To handle various file formats such as CSV, XML, EPCIS
- An interoperable tracing system
- Data exchange protocols such as EDI, SFTP, or APIs
- A system to exchange 3T documentation
Investing in intelligent supply chain software can help you achieve robust serialization, traceability, and aggregation capabilities and prepare you for the final DSCSA deadline.
EXEMPTIONS UNDER DSCSA
Not all medical products fall into the DSCSA jurisdiction. Here are some medical products exempt from the requirements of the Act:
- Intracompany distributions
- Distribution of product samples
- Distribution of over-the-counter drugs
- Medical convenience kits
- Anesthetics, Anti-coagulants, Vasopressor, IV solutions, Sympathomimetics
- Approved animal drugs
- Medical gas distribution
- Blood and blood components for transfusion
If you want to understand more and how to put the systems in place to meet these compliance requirements, our expert will be more than happy to assist you.