
A Better Serialization Solution

A Practical Guide to Carbon Accounting

A Practical Guide to Complying With the EU’s Battery Regulation
Achieving Compliance and Gaining Efficiencies With Optel’s Verify Platform
Achieving Compliance Without Disrupting Production

Advanced Scope 3 Emissions Management

Agricultural Supply Chains: A Traceability Case Study

Bahrain NHRA Regulation timeline

Brazilian Pharmaceutical Company Uses Optel’s Traceability Solution to Meet Compliance Regulations

Bringing Sustainability to the Cocoa Value Chain through Traceability

Combating Counterfeit Premium Spirits with Digital Traceability

Combating Electronic Counterfeiting with Digital Traceability

Combating Illegal Pesticides with Artificial Intelligence

Combating Illegal Production and Eliminating Supply-Chain Deficiencies

Combating Medical Device Counterfeiting with Product Authentication Technology

Countsafe - Slat Counter

Creating Value for the Consumer and Gaining Supply Chain Efficiency
Decreasing Dental Product Counterfeiting with Digital Traceability

Digital Traceability: A Framework for More Sustainable and Resilient Value Chains

Digital Traceability: The Future of Food Safety

Disruptive Technology and the Intelligente Supply Chain

Ensuring Secure Regulatory Compliance and Authenticity

EUDR Guideline

Fighting Counterfeiting While Contributing to the Circular Economy
Flexible and Tailor-Made Solutions to Ensure Compliance

Flexible Solutions to Satisfy Present and Future Customer Needs

Full-Service Serialization Implementation Support

Gain Visibility From Farm to Manufacturer and Mitigate Impact on Amazon Rainforest

Global Life Science Company Uses OPTEL’s L4 Solution for Its Track and Trace

Global Traceability for the Canadian Aluminium

How Galderma Plans on Going Beyond Compliance

Increasing Loyalty Program Membership with Digital Traceability

Increasing Product Security and Supply Chain Visibility

Japanese Beverage Company Conducts LCA With OPTEL Carbon Tracking Solution

Kazakhstan Pharmaceutical Regulation Timeline

Keeping Tabs on Kegs through Digital Traceability

Leveraging End-to-End Traceability to Authenticate the Origin of Primary Materials and Semi-Finished Products

Leveraging Serialization to Mitigate Complexity and Gain a Competitive Advantage

Mitigating Product Diversion with Supply Chain Traceability
Modern Solutions to Tracking Manufacturers’ Carbon Footprints

Optchain by Optel

Optchain for Deforestation-free Supply Chains
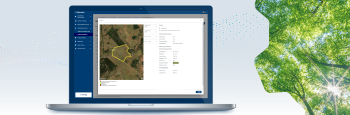
OPTCHAIN for EUDR Compliance
Optchain Inventory Tracking Solution for Real-Time Visibility

OPTEL’s GeoTraceability Solutions Change the Game for Artisanal, Small-Scale Miners (ASMs)

Optimizing Packaging Recyclability and Life Cycle of Plastic Containers

Optimizing Recalls and Returns Management Through Traceability

Overview of European Environmental Regulations and Frameworks

Preventing Counterfeiting and Diversion of Health and Beauty Products

Proving Palm Oil Compliance With EUDR Regulations

The Challenges of Raw-Material Procurement

The Key to Successful GHG Emissions Reporting and Reduction

Tracking and Optimizing the Life Cycle of Reusable Refpet Bottles

Using Digital Traceability to Increase Safety and Regain Trust

Using Traceability to Bring More Visibility to Vietnamese Coffee Production

Uzbekistan Pharmaceutical Regulation Timeline

Virtual Manufacturer Finds Easy L4 Solution

Vision Systems: Typical Applications

What Data Can Optchain Capture
