L4–L5 Track & Trace Platform for Pharma
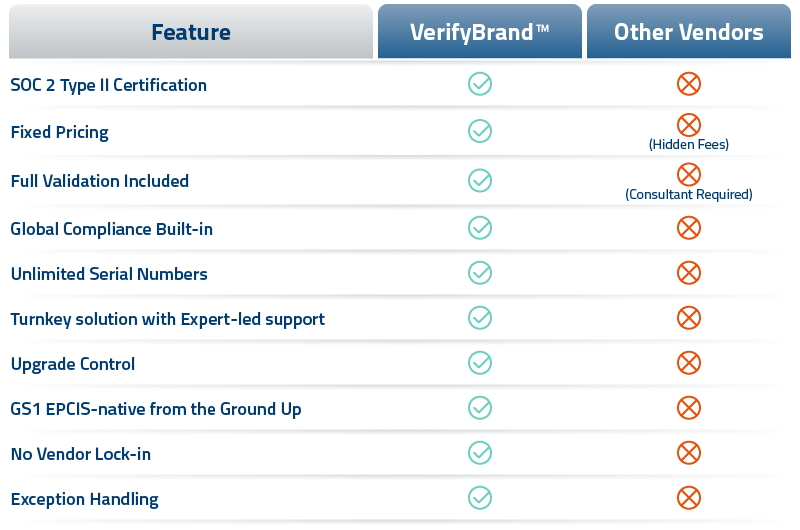
Trusted by top manufacturers worldwide, VerifyBrand™ is fully aligned with global regulations, such as U.S. Drug Supply Chain Security Act (DSCSA), the EU Falsified Medicines Directive (FMD), UAE Tatmeen, Russia’s Chestny ZNAK / Federal Law 425-FZ, and national pharma track-and-trace requirements in China, India, and Central Asia – making it the smart, scalable choice for global pharmaceutical serialization.
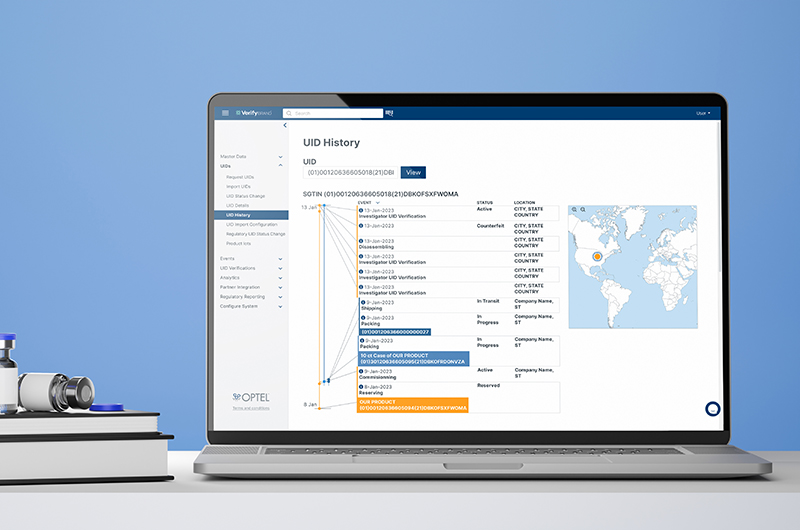
How it works
- Complete L4–L5 serialization system that manages serial numbers, events, and reporting across all your markets.
- GS1 EPCIS-native so you avoid proprietary formats, vendor lock-in, and T2 data issues.
- Real-time validation & exception handling to stop bad data before it becomes a compliance or shipment problem.
What the platform does for you
- Generates unique serial numbers with an extremely low collision rate – even at high volumes.
- Automates aggregation from unit to case to pallet, so every product is traceable.
- Integrates with your ERP, MES, and WMS via standard APIs, without ripping out your existing stack.
- Provides dashboards and alerts so your team can see line status, shipments, and compliance at a glance.