Uzbekistan is taking proactive measures to improve the healthcare standards in the country and developing an efficient traceability system.
In November 2020, the Government of Uzbekistan published Decree 737, which focused on implementing mandatory serialization for pharmaceuticals, alcohol, tobacco, soft drinks, and appliances by 2022. It also establishes that manufacturers and importers are responsible for the labeling and digital marking of products. To that end, the country kickstarted a 6-month pilot project for serialization in 2021.
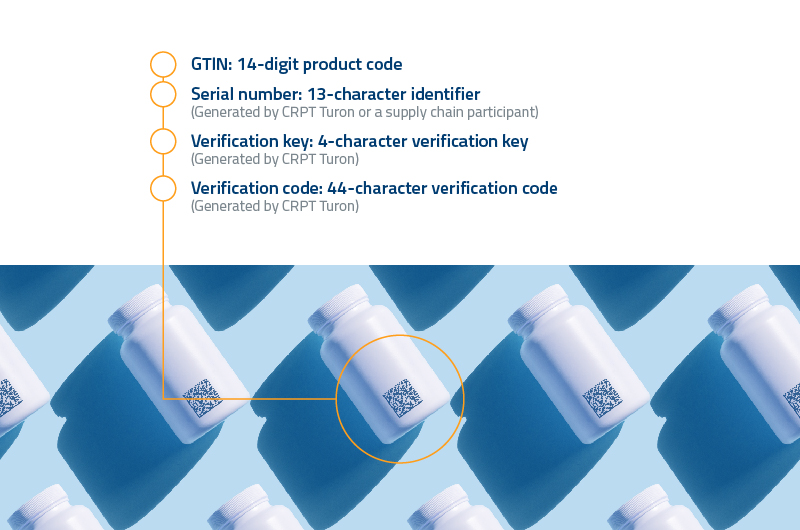
To improve the accounting mechanisms in relation to the production, import and circulation of medicines and medical products, ensure their legal circulation, Cabinet of Ministers of the Republic of Uzbekistan in April 2022 approved a resolution on the phase wise introduction of a mandatory system of digital labelling of medicines and medical devices from 2022-25. It is called decree 149.
According to the decree, starting from September 1, 2022, medicines and medical devices of foreign origin can be imported into the territory of the Republic of Uzbekistan only with the presence of mandatory digital labelling (in secondary packaging). A similar requirement comes into force on November 1, 2022 for medicines in primary packaging.