35
Years of Expertise
6000
Systems Installed Worldwide
25B
Products Tracked Per Year
24 / 7
International Tech Support
96 %
Satisfaction Level Of Service
















































































































Discover our end-to-end traceability solutions

ESG TRACEABILITY
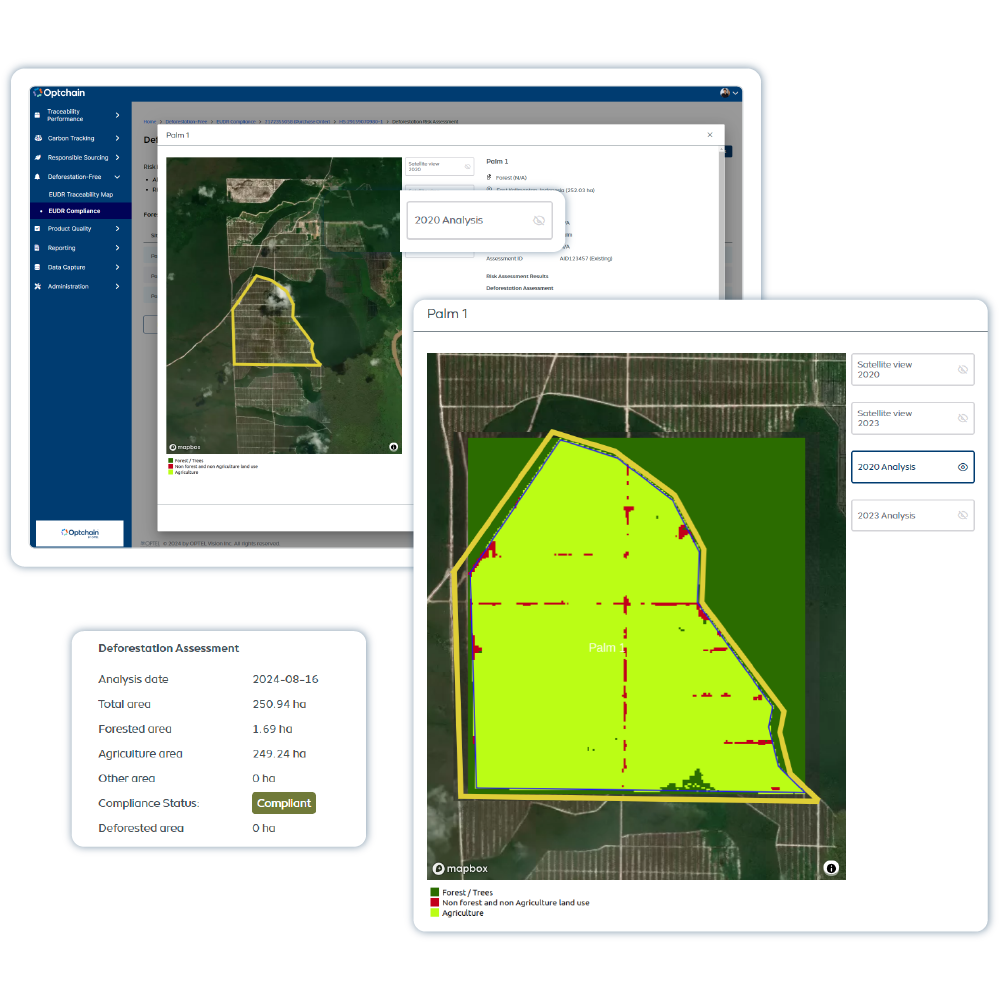
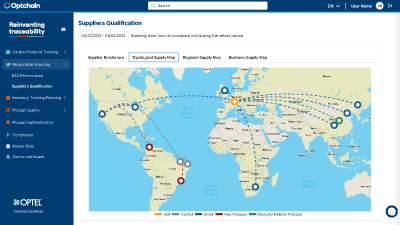
Optchain is a supply chain sustainability platform that ensures ESG compliance and full product life cycle visibility. Its modules cover regulations like EUDR, DPP, CBAM, CSRD, CSDDD and more. Optchain also tracks carbon emissions across supply chains, helping businesses measure, monitor, and reduce their environmental footprint
Discover Optchain™


TRACK & TRACE AND INSPECTION SYSTEMS
Site-level serialization and aggregation hardware solutions to optimize line performance and ensure track-and-trace excellence with AI-integrated vision systems.
Discover TrackSafe

SUPPLY CHAIN INTEGRITY SOLUTION
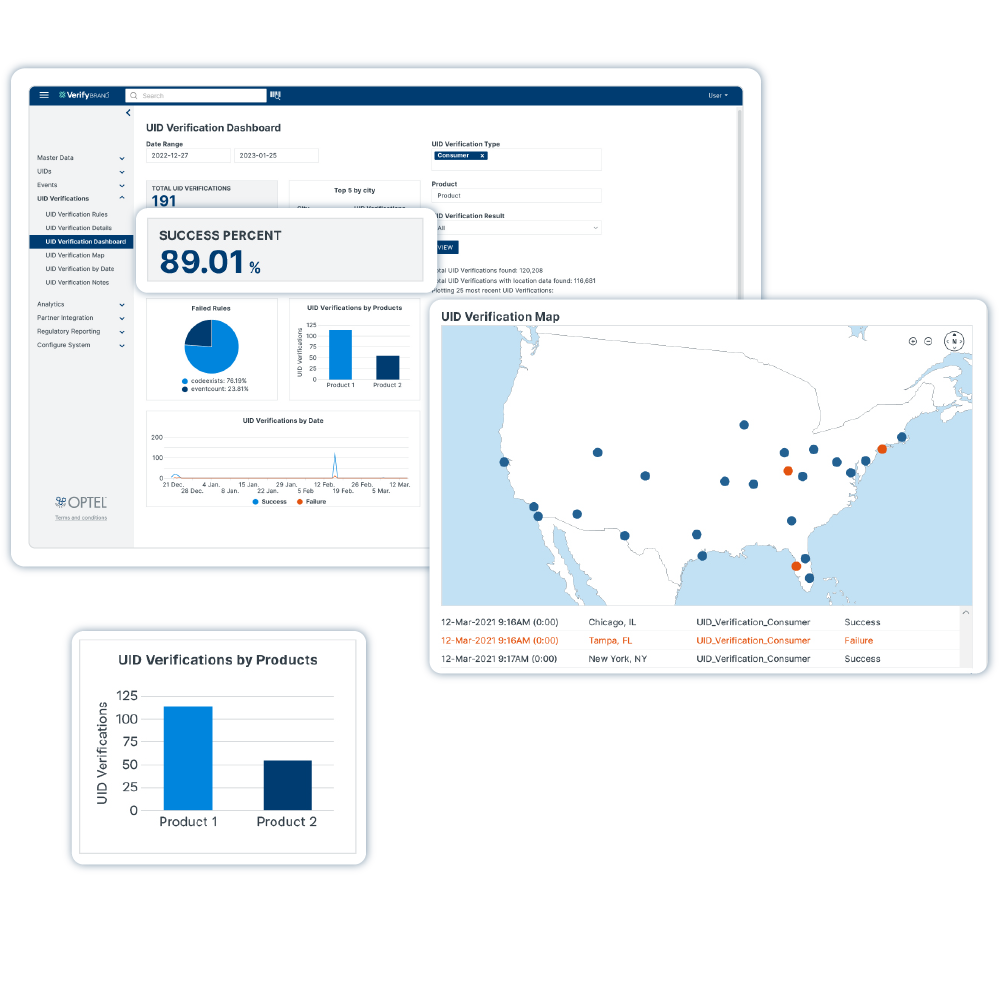
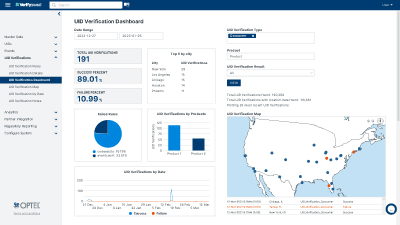
VerifyBrand™ is a real-time serialization and traceability software ensuring compliance, enhancing brand protection, authenticating products, and optimizing inventory.
Discover VerifyBrand™