Cultural DNA Story
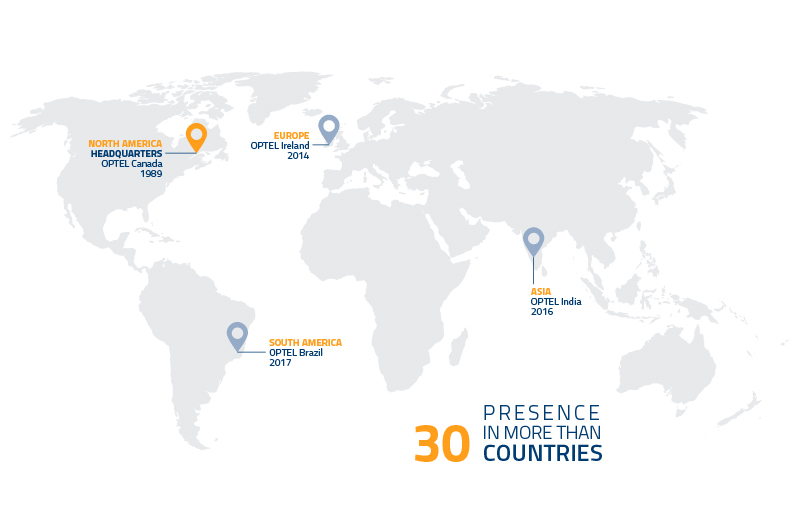
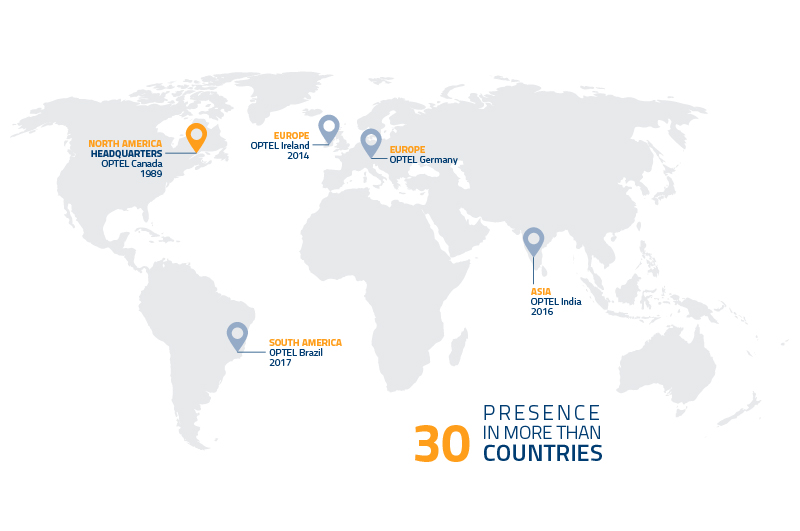
OPTEL finds the solutions to today’s biggest problems: climate change, secure healthcare and sustainable living. How? By developing intelligent supply chains made possible through advanced traceability technologies. For the last 30 years, OPTEL has partnered with the world’s top brands who are dedicated to using the power of business to do good in the world. Today, with systems present in over 30 countries, OPTEL is known as a socially and environmentally responsible leader in transformative technology powered by the passionate thought leaders of our time. Just like the industries they work in, OPTEL moves quickly and embraces change. This is a place where company culture comes naturally because the team has a common core objective: