35
Anos de Experiência
6000
Sistemas Instalados em Todo o Mundo
25B
Bilhões de Produtos Rastreados por Ano
24 / 7
Suporte Técnico Internacional
96 %
Nível de Satisfação de
















































































































DESCUBRA NOSSA SOLUÇÃO DE RASTREABILIDADE DE PONTA A PONTA

Rastreabilidade ESG
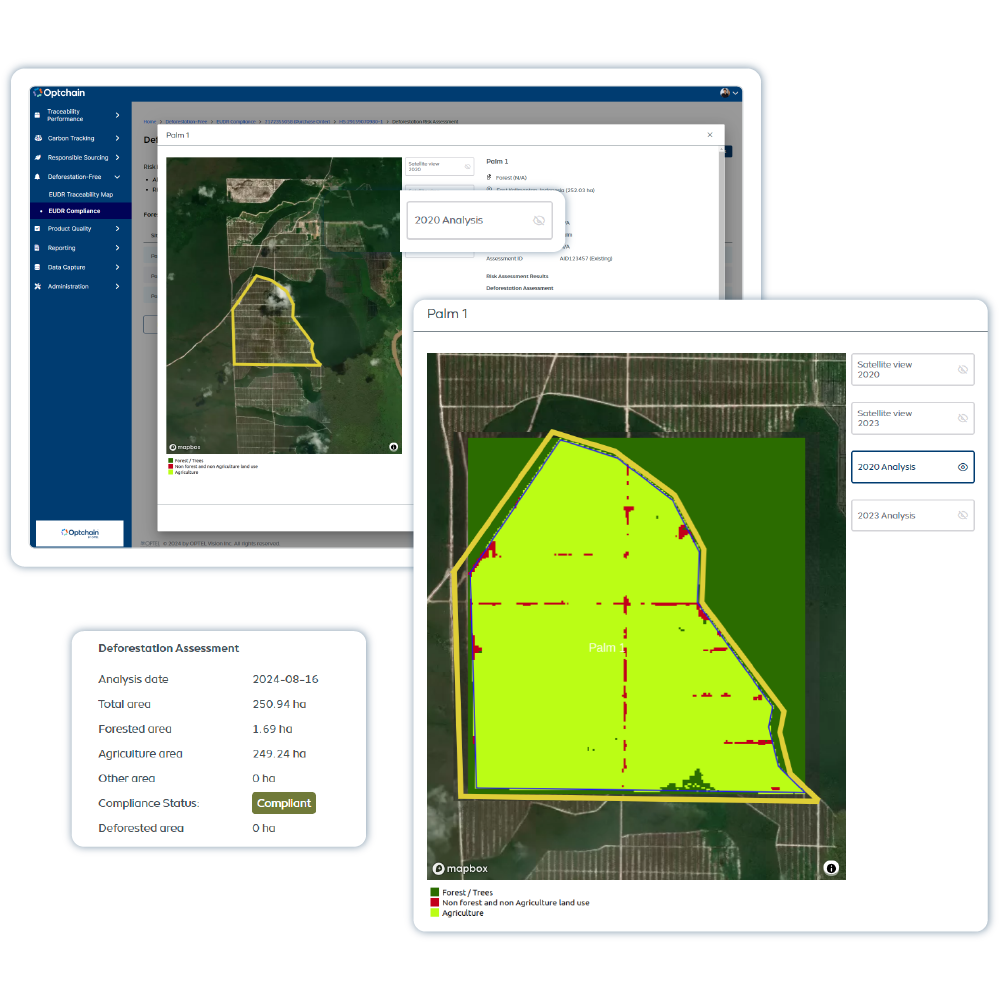
Optchain é uma plataforma de sustentabilidade da cadeia de suprimentos que garante conformidade ESG e visibilidade total do ciclo de vida dos produtos. Seus módulos cobrem regulamentações como EUDR, DPP, CBAM, CSRD, CSDDD e mais. O Optchain também rastreia as emissões de carbono nas cadeias de suprimentos, ajudando as empresas a medir, monitorar e reduzir sua pegada ambiental de acordo com os objetivos de sustentabilidade.
Descubra o Optchain


Sistemas de Rastreamento e Inspeção
Soluções hardware de serialização e agregação para otimizar o desempenho da linha e garantir excelência em track & trace com sistemas de visão integrados à IA.
Descubra o TrackSafe

Solução de Integridade da Cadeia de Suprimentos
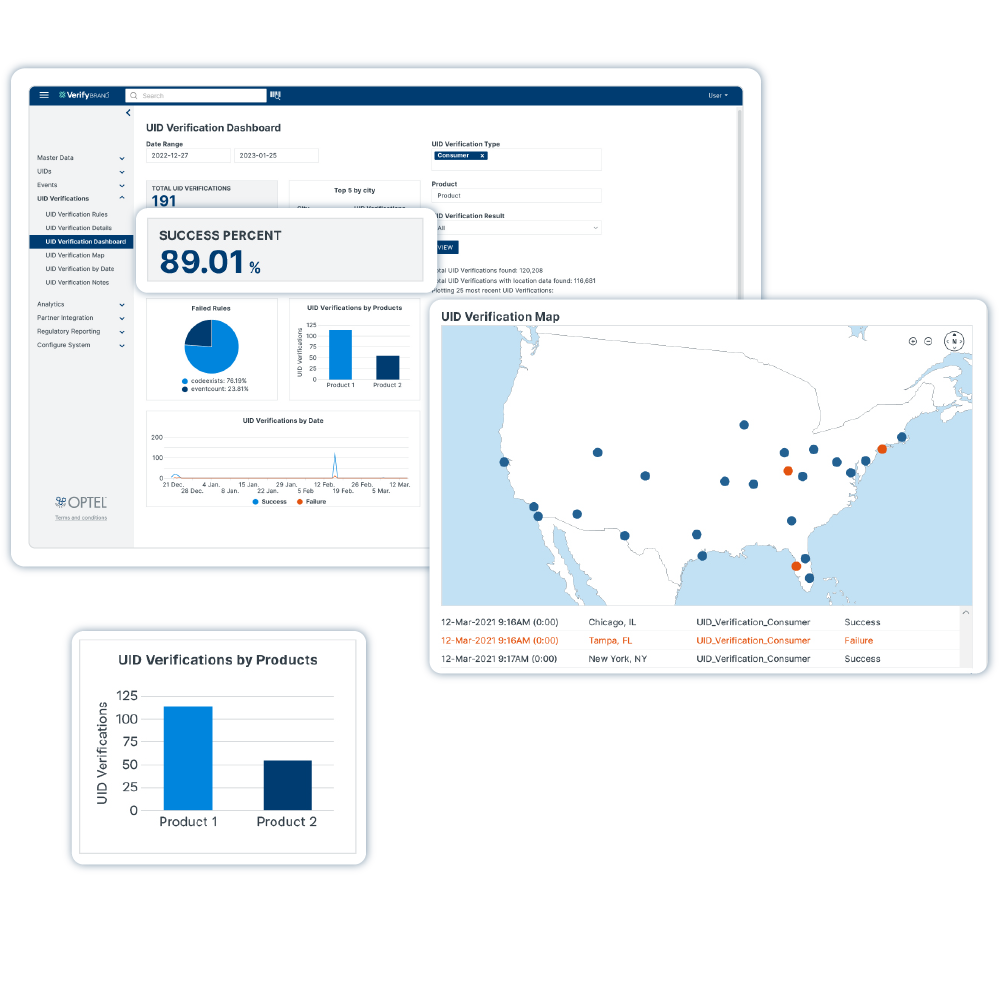
VerifyBrand™ é um software de serialização e rastreabilidade real que garante conformidade com regulamentações, aprimora a proteção da marca, autentica produtos e otimiza o estoque.
Descubra o VerifyBrand™