35
Années d’expertise
6000
Systèmes installés dans le monde entier
25B
Milliards de produits suivis par an
24 / 7
Support technique international
96 %
de satisfaction chez notre clientèle
















































































































DÉCOUVREZ NOTRE SOLUTION DE TRAÇABILITÉ DE A À Z

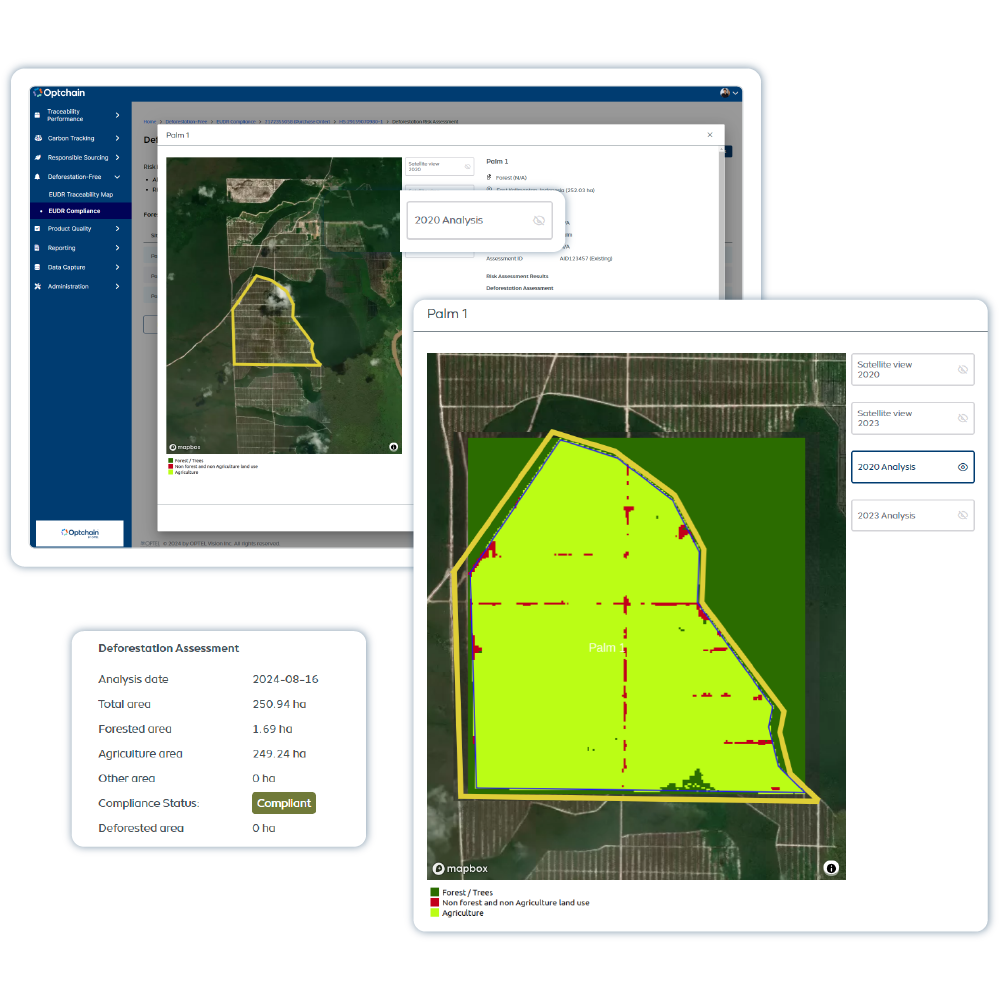
Traçabilité ESG
Optchain est une plateforme de gestion durable des chaînes d’approvisionnement, offrant une visibilité complète sur le cycle de vie des produits et garantissant leur conformité ESG. Ses modules permettent de se conformer aux exigences de réglementations telles que l’EUDR, le DPP, le CBAM, le CSRD, le CSDDD, et bien d’autres. Optchain suit également les émissions de carbone tout au long des chaînes d’approvisionnement.
Découvrez Optchain


Systèmes de Suivi, de Traçage et D’inspection
Solutions matérielles de sérialisation et d’agrégation au niveau du site pour optimiser les performances de ligne et garantir l’excellence du suivi et du traçage avec des systèmes de vision intégrés à l’IA.
Découvrez TrackSafe

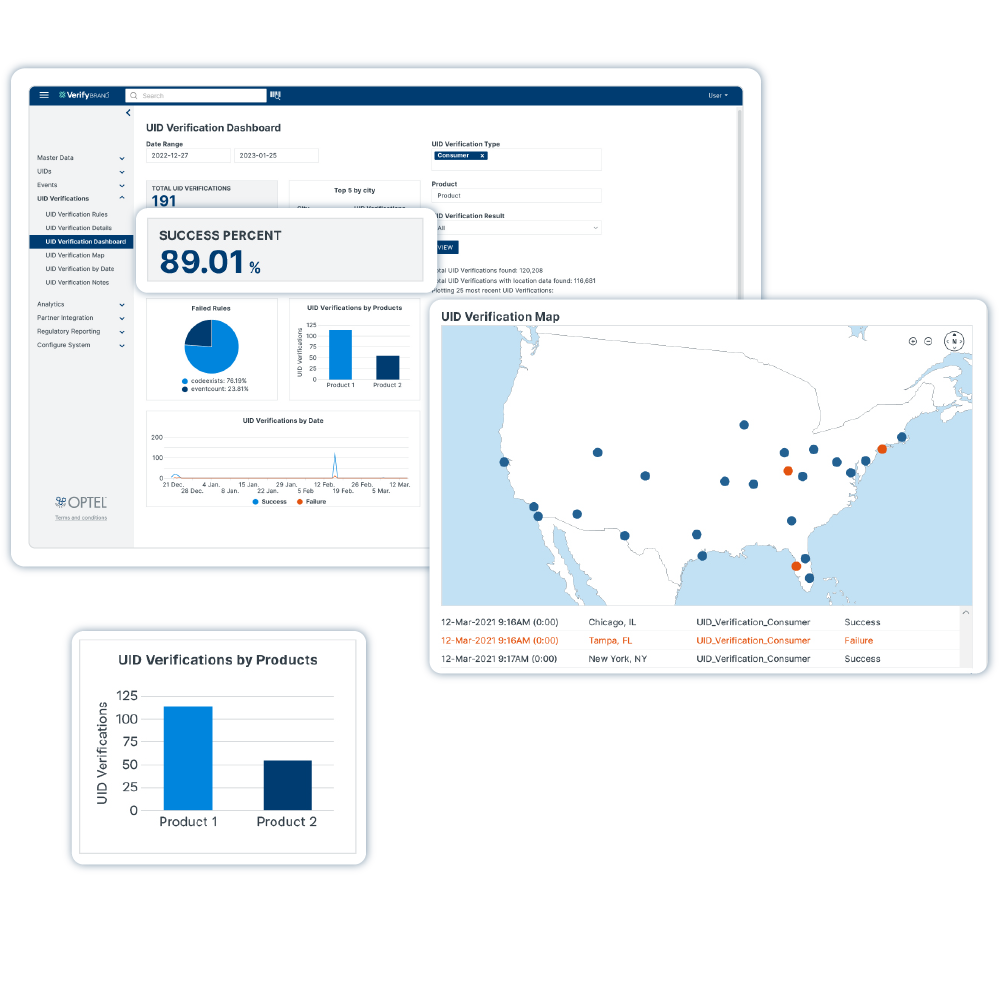
Solution d’intégrité de la chaîne d’approvisionnement
VerifyBrand™ est un logiciel de sérialisation et de traçabilité en temps réel garantissant la conformité, renforçant la protection de la marque, authentifiant les produits et optimisant les stocks.
Découvrez VerifyBrand™