LineMaster
Software embedded in the line that records all serialization and aggregation events at the packaging level
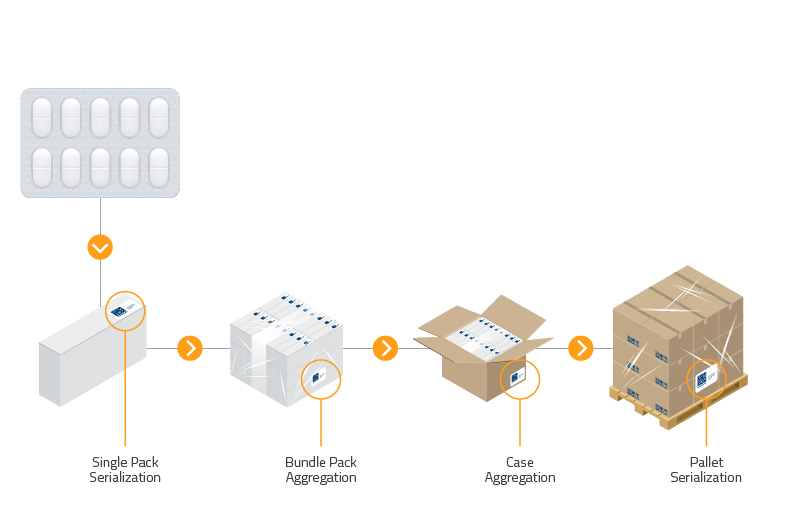
If serialization was the beginning of pharmaceutical track and trace then aggregation isn’t the end but it’s the next logical step to enhance supply chain security and traceability.
Those choosing aggregation today are moving ahead of their competition in terms of efficiency, security and traceability while removing many barriers to new markets.
While aggregation is key to patient safety and therefore a secure pharma industry, this process comes with new challenges.
These are the reasons why you need to choose your aggregation provider wisely and make sure they provide true expertise and support during the implementation process.
Many pharmaceutical companies are experiencing cost savings from improvements along with the following:
Discover Intelligent Supply Chain


LineMaster
Software embedded in the line that records all serialization and aggregation events at the packaging level

Kompano
Solution that manages the serialization of products in the warehouse: prints labels, reworks, sending and receiving

Semi-Automated Print Station
The Semi-automated Print Station: Fastest Solution to implement Serialization and Aggregation for low volume production.

PackStation (FMP)
Manual aggregation solution that can also serialize any level of packaging

PackStation (SAP)
Semi-automatic solution designed to read serial numbers and add them to shipping cartons
Offline LabelTracker
Solution that prints and inspects labels off the production line while still meeting compliance requirements
CLTracker (TE)
Solution developed for implementation in sealing units or tamper-evident features that serializes and inspects data
CartonTracker
Automated serialization solution with great stability for printing and inspection, and that has an integrated ejection system
BottleTracker
Solution that inspects and adds bottle labels without interrupting the production flow
BundleTracker
Solution that makes the aggregation of grouped products. It checks product count and status while rejecting defective packages

PalletMaster
Aggregation solution that identifies and checks boxes and pallets with cameras and/or barcode readers for the central palletizing area

Association Station
Solution that checks serialized products and facilitates aggregation into secondary packages

Vision Systems
OPTEL’s vision systems help ensure quality and safety

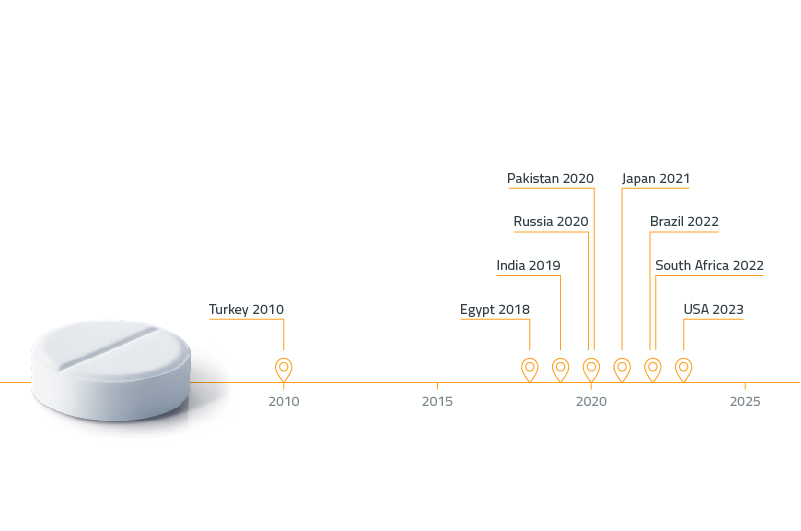
Serialization
Serialization is the first step in the traceability journey

Site-Level Solutions (L3)
Manage your plant-level serialization operations with the latest technology

Enterprise-Level Cloud (L4)
Optchain Verify is an essential tool to comply with most of the world’s pharmaceutical regulations
